Atoms
Atoms are the building blocks of ALL things. There are actually many things smaller than atoms, but we only care about 3 of those things. Neutrons, protons, and electrons.
In this section you’ll learn about what neutrons, protons, and electrons are, their basic properties, and how the interact within an atom!
Table of contents
The Atom
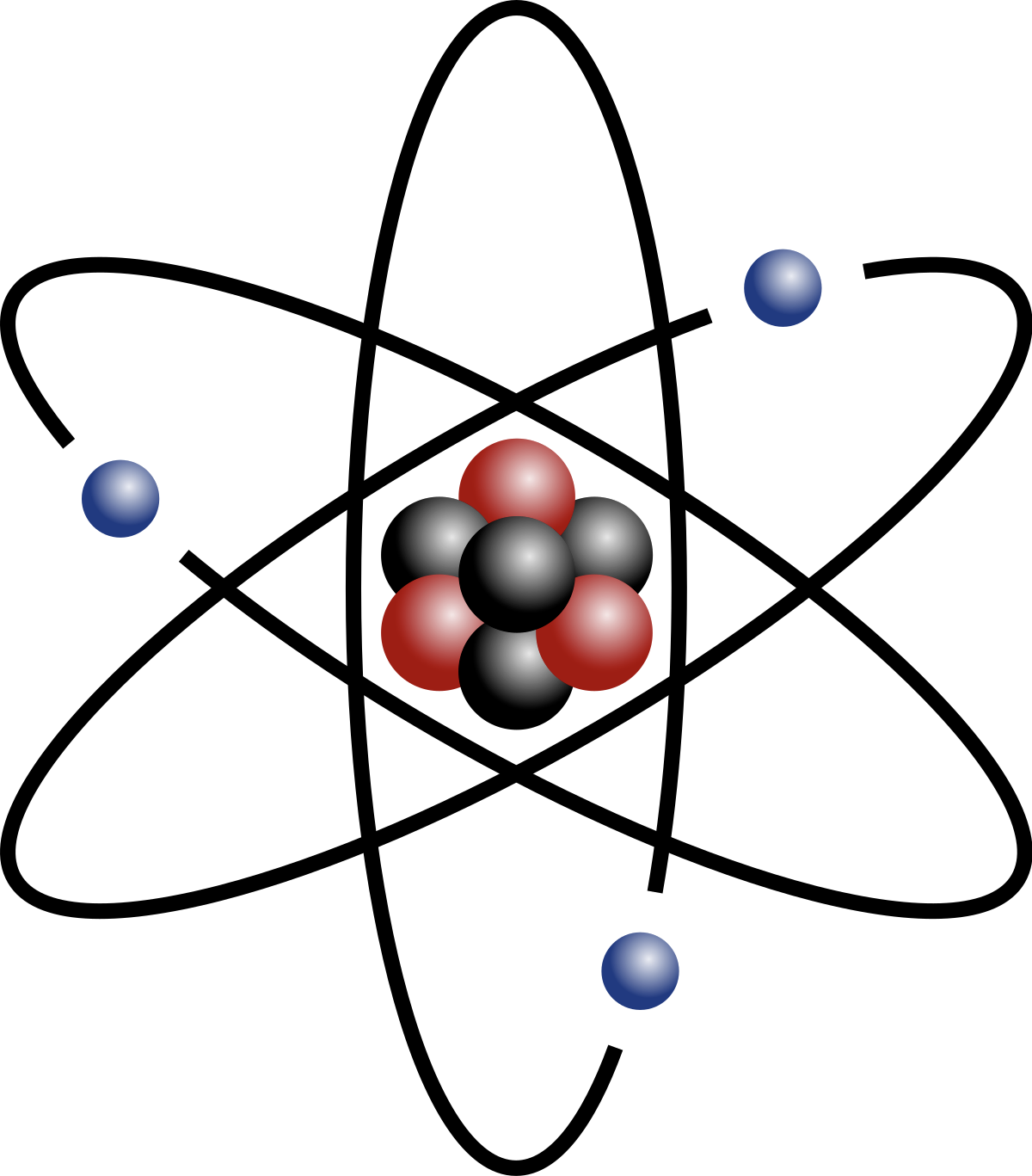
The atom is the building block of all things. There are three main parts that make up an atom, neutrons, protons, and electrons. The following table summarizes the properties of each of the three parts.
| Particle | Mass | Charge | Location |
|---|---|---|---|
| Neutron | 1 u | 0 or none | Nucleus |
| Proton | 1 U | +1 | Nucleus |
| Electron | \(\approx 0\) | -1 | Outside the nucleus |
There are two parts to an atom, the nucleus, or center of the atom, and the outside. The nucleus is where nearly all of the mass is located in an atom because that is where the protons and neutrons are. The outside part looks like a cloud, because that is where the electrons are flying around.
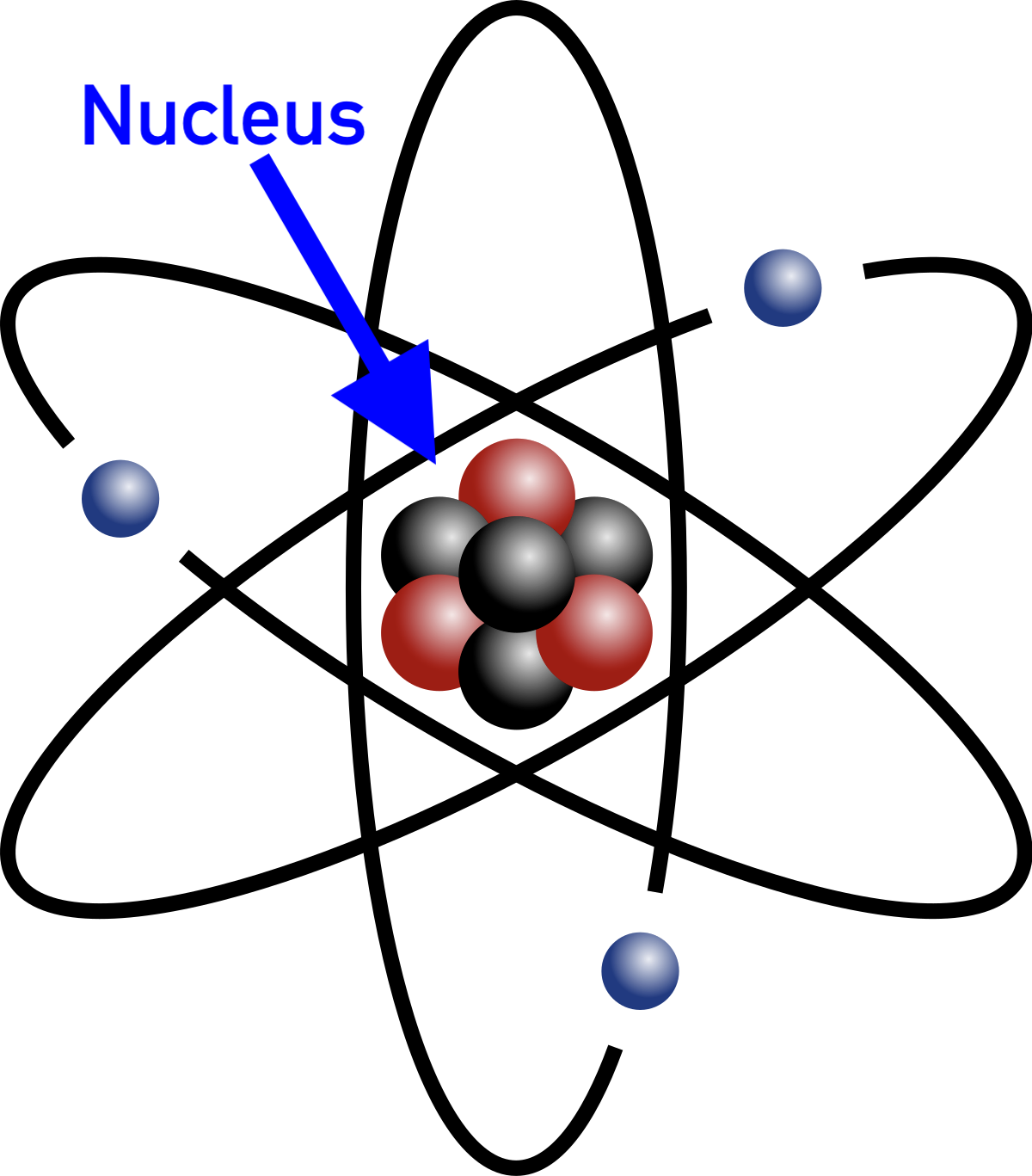
Proton
Protons are what determine the element. The number of protons is the same as the atomic number (see the section on the periodic table). All of the protons live in the nucleus or the very center of the atom. Below is a table of their basic properties.
| Mass | Charge | Location |
|---|---|---|
| 1 u | +1 | Nucleus |
A ‘u’ is the atomic mass unit. 1u is \(1.66\times10^{-27}\) kg. It is such a small number that it is much, much easier to use atomic mass units than grams. It also makes all the math easier because both protons and neutrons have a mass of 1 u.
Neutrons
Neutrons are the glue that hold the nucleus together. They act as ‘spacers’ to keep the protons separated just enough that the nuclear forces can overcome the electric forces that are trying to push the protons apart.
| Mass | Charge | Location |
|---|---|---|
| 1 u | 0 or none | Nucleus |
Isotopes
Protons determine the element, but neutrons determine the isotope. An isotope is an element that has the atomic mass specified, ‘[Element]-[Atomic Mass]’. If no number is specified, it is assumed that you are referring to a bunch of atoms, the element. If you specify the atomic mass you are referring to an individual atom, or a collection of homogeneous atoms (atoms that are the same).
Examples:
- Carbon is a element.
- Carbon-12 is an isotope that has an atomic mass of 12.
- Carbon-13 is an isotope that has an atomic mass of 13.
- Carbon-14 is an isotope that has an atomic mass of 14.
In nature, isotopes occur in certain percentages depending upon how stable a given isotope is.
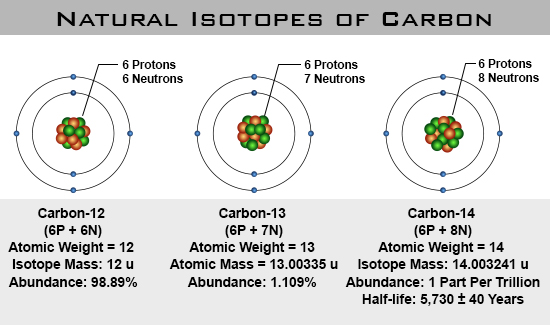
As seen above carbon comes in 3 types; Carbon-12, Carbon-13, and Carbon-14. When referred to as Carbon-12, just the atom that has an atomic mass of 12 is being referred to. When referred to as carbon it means that you have a mixture of all carbon isotopes in a given bulk material.
Application - Carbon Dating
When alive, living things are constantly turning over the carbon in their bodies with the carbon in their environment through respiration (breathing) and the food they eat. When living things die the carbon getting exchanged with their environment. This means that when alive, all things have the same percentage of carbon-14 as their environment.
So when something dies the carbon-14 starts to decay because it is radioactive, meaning it is unstable and will spontaneously break down at a known rate. We can then determine how old something is by how little carbon-14 is left when we examine it.
Electrons
Electrons are tiny (relative to protons and neutrons), negatively charged, sub-atomic particles that fly around the outside of every atom. Because electrons are on the outside, they are what interact with everything, so the electron determine how the atom interacts with things.
| Mass | Charge | Location |
|---|---|---|
| \(\approx\) 0 u | -1 | Outside |
Energy Levels
There are two things you need to know about electrons, they don’t share their space, and they all want to be as close to the nucleus as possible. So:
- Electrons exist in different energy levels because they cannot share space.
- Electrons fill from the lowest or closest energy level outward because they want to be as close as possible.
One last thing about electrons. Electrons are directionally challenged, meaning that if one travels left, and one travels right, then they don’t interfere with each other. This means that 2 electrons can fit into each energy level, hence the 2 in the equation below. Long story short, each orbital n, can hold:
\[\text{Electrons per orbital n} = 2n^2\]The first orbital can hold 2, the second can hold 8, the third can hold 18, and so on.
The simplest way to show the orbitals or energy levels is to have a central nucleus with concentric rings representing the electron orbitals.
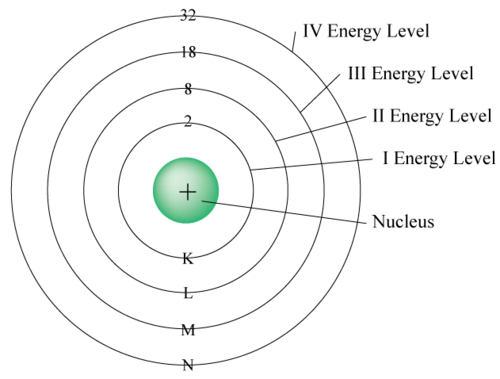
This model shows the concept of energy levels very well but does not represent the actual locations of electrons. In the current model the energy levels are called orbitals because the locations of the electrons is not spherical. Below is the current model, note how the final result is that the electrons are packed as closely as possible to each other and the nucleus.
Valence Electrons
Valence electrons are the outermost electrons. They are the face that the atom presents to the world and are thus how the atom interacts with the world. Because the electrons move so fast, the ones on the outside effectively hide or shield the outside world from seeing all the other electrons inside.
The tall columns of the periodic table, the first two and last six, indicate the number of valence electrons. The first column has 1 valence electron, the second has 2, and so on, until the very last column has 8. Helium is the one exception because the first orbital can only hold two atoms.
The ultimate goal of any atom is have a full set of valence electrons. Atoms will do ANYTHING to have a full set of valence electrons. Stealing or giving away electrons as needed. It is easier to eject electrons if the atom is in columns 1, 2, 13. It is easier to steal electrons if the atom is in column 15, 16, 17. If it is in the middle, column 14, it can go either way. If it is happy and content, column 18, then the atom will ignore everyone that passes by.
Ions
Ions are any atom that have a non-zero charge. All atoms are neutral by nature, but things do happen.
No matter what happens to the electrons, the nucleus retains the same number of protons. That means that if the atom throws away an electron, the atom has a net positive charge.
Example 1: Lithium is in the first column, so it wants very badly to throw away its extra electron so that it has 2 valence electrons. When it does throw that electron away it has 3 protons and 2 electrons. Hence the charge is now 3(+1) + 2(-1) = +1. The atom is positive.
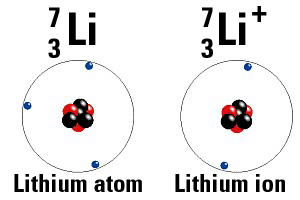
Ions form when either radiation knocks an electron out, or when atoms steal or give away electrons.
If an atom gives up an electron (columns 1, 2, 13) it becomes a positive ion called a cation. If an atom steals an electron (columns 15, 16, 17) it becomes negative and becomes an anion. The easiest way to remember which is which is to look at the prefix ‘a’ in anion. When used as a prefix ‘a’ means ‘without’. Other examples are; amnesia, without memory; amoral, without morals; amorphous, without shape; apathetic, without feeling. Anions are without ‘an ion’ meaning they have given up an electron and are now positive.
Ionic bonds
When a neutral sodium atom sees a neutral chlorine atom, they make a trade. The sodium atom is in column 1 so it really wants to give its extra electron away. The chlorine atom is in column 17, so it really wants an electron. It is a win-win deal. Once they trade though, the sodium ion is positively charged, and the chlorine ion is negatively charged. The same electric forces that act within the atom now act between the two ions and pull them together forming NaCl, table salt.