The Metric System
America is one of the three countries (Liberia and Myanmar are the other two) that do not officially recognize the metric system. By now you have bee using the metric system for years in your science classes so we would like you to think about if we should adopt the metric system and why we have not.
In your lab notebook, answer the following two questions: Do you think America should adopt the metric system (explain why)? Why do you think America has not yet adopted the metric system?
Table of contents
The Metric System
The metric system (also known as the SI system (stands for ‘system international’, it was created by the French)), is a set of units that are used to describe all quantities that we measure in science. EVERY quantity that we can measure.
There was a problem, as scientist came up with new things to measure, each thing had its own unit that was un-related to all other units, or worse, scientists would give them random values simply because they liked them. The conversions were also terrible, but you are familiar with that. 12 inches in a foot, 3 feet in a yard, 5280 feet in a mile, 16 ounces in a pound, 8 fluid ounces to a cup, 16 cups to a gallon, the list goes on. Imagine doing all of these conversions in the days pre-dating calculators!
Thus the metric system was born.
Every unit in the metric system has two parts, a prefix and a base, and is always written as ‘prefix-base’. The base is what is being measured, length, volume, temperature, and so on. The prefix is how big the thing that is being measured is. Rather than have a different name for different lengths (like inch, foot, mile) only the prefix changes.
The Bases
The truly amazing thing is that the metric system only has 7 units. 7. All other units are combinations of those 7 and have been given names only because they are really, really common. In this class you will only use some of the 7, and they are so common you should know most of them already.
| Symbol | Name | What it measures |
|---|---|---|
| m | meter | length |
| s | second | time |
| g | gram | mass |
| A | ampere | electric current |
| K | kelvin | temperature |
| mol | mole | amount of a substance |
| cd | candela | how bright something is |
And here are the most common combinations that you will run across.
| Symbol | Name | What it measures | Equivalent combination |
|---|---|---|---|
| L | liter | volume | \(m^3\) |
| \(\rho\) | – | density | \(\frac{g}{cm^3}\) |
| N | newton | force | \(kg\cdot m /s^2\) |
| J | joule | energy | \(N\cdot m\) |
| W | watt | power | \(\frac{J}{s}\) |
The Prefixes and Conversions
The prefixes set the scale of what is being measured. Does it make sense to use the same unit to measure the width of a hair and the distance to the moon? NO! What they came up with was a set of letters that all represent a specific power of 10. That way when combined with the base you can describe completely different lengths. Without further delay, here they are!
| Symbol | Name | Decimal | Exponent |
|---|---|---|---|
| G | giga | 1,000,000,000 | \(10^9\) |
| M | mega | 1,000,000 | \(10^6\) |
| k | kilo | 1,000 | \(10^3\) |
| – | – | 1 | \(10^0\) |
| m | milli | 0.001 | \(10^{-3}\) |
| \(\mu\) | micro | 0.000 001 | \(10^{-6}\) |
| n | nano | 0.000 000 001 | \(10^{-9}\) |
As you can see all of those vary by factos of 1000. There is one prefix that is common in America that does not fall on that scale and it is c, centi, 0.01, \(10^{-2}\). This is because a cm (centi-meter) is the closest metric unit to an inch.
Here is a visualization method that can help with coverting prefixes.
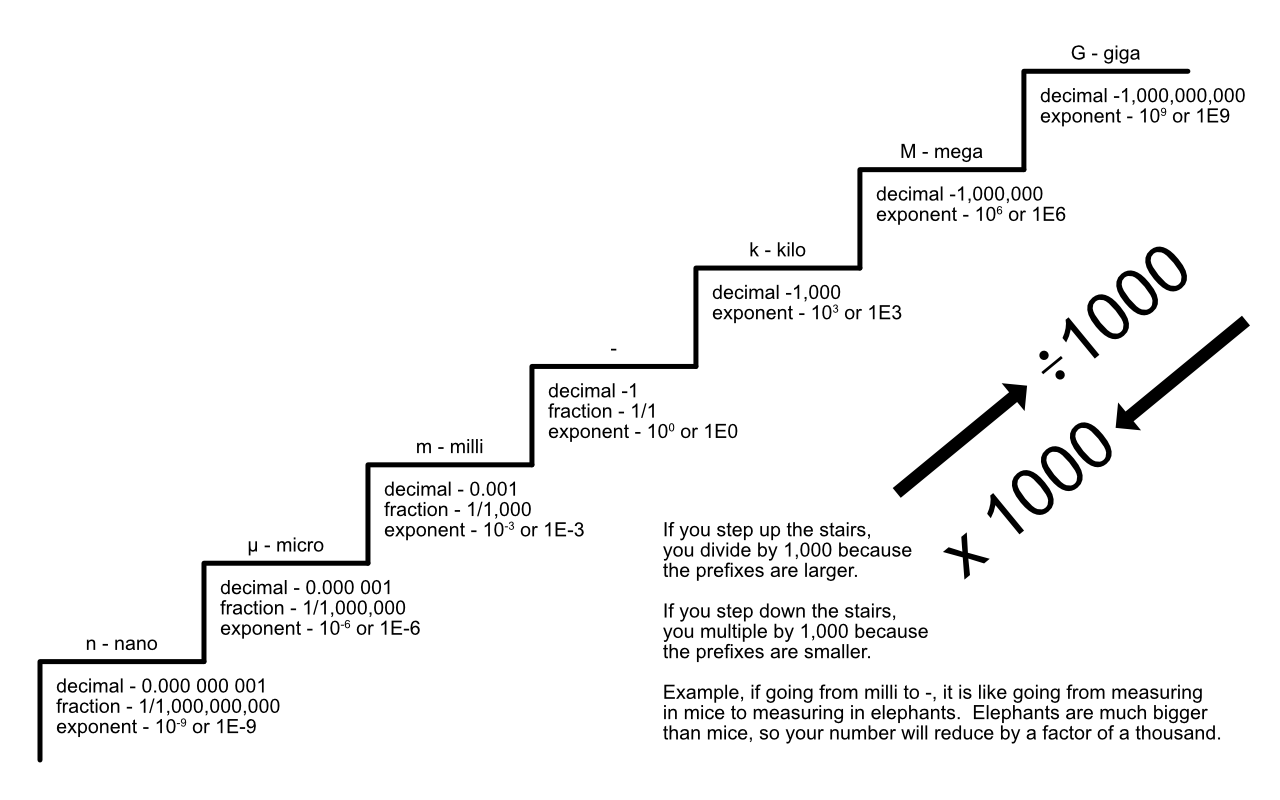
Here is a video that helps you understand just how much can change with each power of 10. IBM Powers of 10
Here is the wonderful part about powers of ten. All the numbers you know are powers of ten, that is how the number system was designed. What this means, and you should know this, when you multiple or divide by 10, the decimal moves around, but the numbers DO NOT CHANGE!
Here is an example. 123 m is 0.123 km. Meters all by it self (m) has no zeros, kilo (k) has three (it is 1,000), so the decimal moves three places when changing from m to km. The question is which way? I always remember it as if the unit gets larger, the number gets smaller. So from meters to km, the unit gets larger, so the number gets smaller. Or if the unit gets smaller, the number gets larger.
Now, there are many tools and tricks to help you with conversions but the best way is to really just practice. Go here for unlimited practice problems. There are some prefixes that we will not use in this class, but it can’t hurt to know them!
Activity - Standards
Units are useless if they don’t mean anything to you. That is the current problem with getting the metric system adopted in the US, is that very few people have any sense of what metric units measure. You are going to fix that problem.
Copy down the table below into your lab notebook. Make it large enough that you can write in each square.
| G | M | k | – | m | \(\mu\) | n | |
|---|---|---|---|---|---|---|---|
| m (meter) | 1m \(\approx\) Length from nose to finger tips | ||||||
| g (gram) | 1 g \(\approx\) paper clip | ||||||
| s (second) | 1 s \(\approx\) tick of a clock | ||||||
| K (Kelvin) | 1 K \(\approx\) 2 degrees Farenheight | ||||||
| L (liter) | 1 L \(\approx\) a ‘big’ water bottle | ||||||
| N (newton) | 1 N \(\approx\) the weight of 1 apple | ||||||
| W (watt) | 5 W \(\approx\) a cell phone charger |
Then your group will pick a column (a base unit) and come up with a ‘standard’ for that prefix-base pair. We’ve given an example for the ‘–’ prefix units. For each of your standards you want it to always be approximately 1, that way it is easy to tell what they measure. We cheated and went with 5 for watts because a cell phone charger is so common.
Activity - Measures Lab
There are four stations that you need to measure various objects. At each station, record the requested measurement into your lab notebook. Once you are done, put all of your data into this form
Length
- Book
- Penny
- Table
Volume
- Cup
- Vial
- Bottle
Temperature
- Boiling water
- Room temperature water
- Ice water
- Ice alcohol
Mass
- Penny
- Block
- 20mL of Water
Activity - Mini Metric Olympics
Your teacher will give you the handout and supplies.
Prep
- Measures lab - 4 stations
- Print station instructions
- Mini-olympic activity - handouts, stations, etc setup